A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. Which ph range describes strong acids.

The Ph Scale Chemistry For Non Majors
The distribution mains are designed for.

. PH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. 7 Which of the following pH values represents the strongest acid. Two drinks P and Q gave acidic and alkaline reactions respectively.
Among given p K a values D is the lowest p K a value. Bases have many uses. Water that has more free hydrogen ions is acidic whereas water that has more free hydroxyl ions is basic.
What might be its pH. Hydrochloric acid is the strongest acid on the pH scale. You suspect that a chemical that you are testing in the lab is strongly acidic.
You suspect that a chemical. The pH scale which measures from 0 to 14 provides an indication of just how acidic or basic a substance is. What is 7 on the pH scale.
The pH value of an acid represents its __________ of positive ions in the solution. Thus measured pH values will lie mostly in the range 0 to 14 though negative pH values and values above 14 are entirely possible. This is considered a strong acid because it dissociated completely in solution to form H and Cl- ions.
9th - 12th grade. The pH scale ranges from _____. Standard EDTA ethylene diamine tetra acetic acid solution.
A large Ka value also means the formation of products in the reaction is favored. Further the acidity decreases as the value of pH increases from 0 to 7 whereas solutions with the value of pH equal to 14 are termed as strongly basic solutions. If foreign strong substances dramatically change this pH our bodies can no longer function properly.
Which of the following is a property of a base. The higher the number the stronger the base. On the pH scale 15 and 83 and 6 which would be the strongest acid.
Which of the following pH values represents the strongest acid. The higher the concentration of negative hydroxide ions in the solution the stronger the base is. Acids and Bases DRAFT.
0-6 is acidic 7 is neutral and 8-14 is alkaline. A solution of a strong acid such as hydrochloric acid at concentration 1 mol dm 3 has a pH of 0. Of pH value 7.
The range goes from 0 - 14 with 7 being neutral. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base. One has a pH value of 9 and the other has a pH value of 3.
Which drink has a pH value of 9. Which of the following values of pH represents a stronger acid. A solution of a strong alkali such as sodium hydroxide at concentration 1 mol dm 3 has a pH of 14.
The basicity decreases as the value of pH decreases from 14 to 7. All bases have a sour taste. Lemon juice - 25.
Which of the following is the strongest acid. A sodium B oxygen C hydrogen D nitrogen E carbon. See Page 1.
What is 7 on the pH scale. Which of the following pH values represents the strongest acid. The Ka value for most weak acids ranges from 10-2 to 10-14.
Baking soda and antacid tablets are examples of two common _____. Arrange the following in the increasing order of acidic strength. Oven cleaner - 135.
A 2 b 5 c 7 d 10. Acid is 0-3 lower numbers. The pH scale ranges from _____.
Which of the following pH values represents a base. Which of these pH values represent an acid. So Acid with p K a value 1 0 2 is the stongest acid.
A 4 B 10 C 2 D 7 E 12. 8 Of the following elements which is the least common in living organisms. They are mainly used to make.
You can prepare a solution of HCl with pH 60 if the molar concentration of. 9 In ionic bonds A electrons are shared unequally between atoms B electrons are shared equally between. A small Ka value means little of the acid dissociates so you have a weak acid.
The strongest acid will have the lowest pH if. Most parts of our body excluding things like stomach acid measure around 72 and 76 on the pH scale a 7 is neutral on the scale. Solutions having the value of pH equal to 0 are known to be strongly acidic solutions.
Possibly the strongest acid that we come into contact with is hydrochloric acid. Hence Option D is the correct answer. If I have a substance with a pH of 3 what is it.
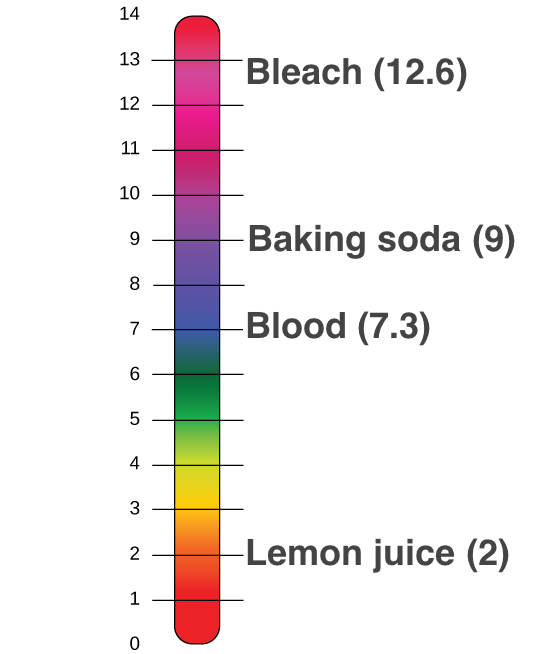
Ph Acids And Bases Review Article Khan Academy

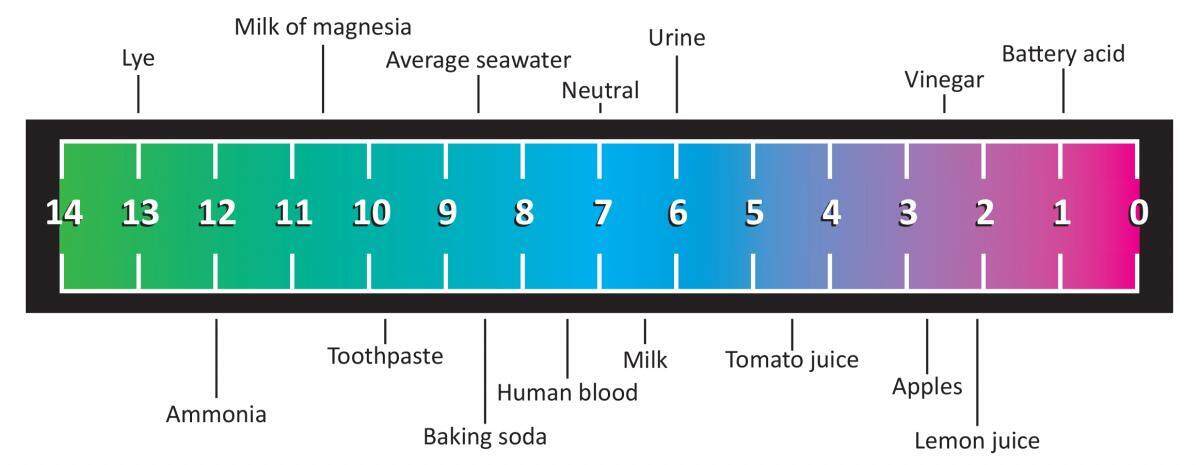
0 Comments