And a d subshell l 2 consists of five orbitals called d orbitals. Here the energy level n4.
How Many Electrons Are In Each Shell Including 3p Orbitals
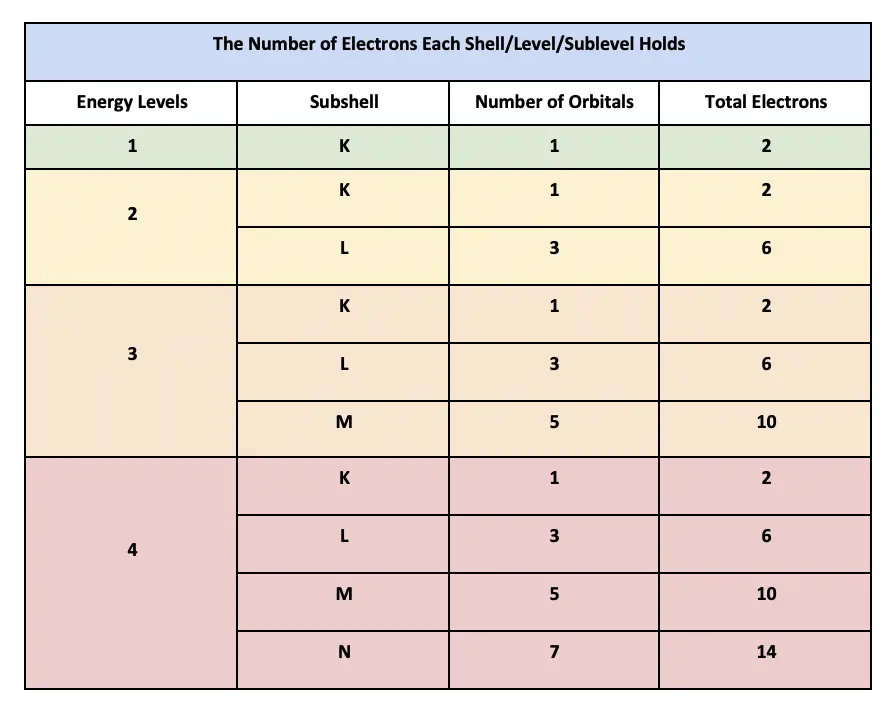
The s subshell which has 1 orbital with 2 electrons the p subshell which has 3 orbitals with 6 electrons the d subshell which has 5 orbitals with 10 electrons and the f subshell which has 7 orbitals with 14 electrons for a total of 16 orbitals and 32 electrons.

. Here is an easy formula for it though it isnt necessary max number of electrons a subshell can hold2 of orbitals in that subshell. 22 How many sublevels are in the 7th energy level. Three possible orientations - There are five possible orbitals in a d subshell and 7 possible orbitals in an f subshell.
13 How many possible orbitals are there in n 4. Five orbitalselectronic configuration. These wave functions have 3 representations described by the intergers ml of 1 0 and -1 which are the orbitals of the p subshell.
6 How many orbitals are in p shell. The fourth shell has 4 subshells. We review their content and use your feedback to keep the quality high.
14 Is 4s more penetrating than 3d. On adding all the orbitals of 3s 3p and 3d we get a total of 9 orbitals. In an atom what is the maximum number of electrons that can have a principal quantum number n 6.
Because each orbital correspond to a specific value of orbital angular momentum azimuthal quantum number. 11 How many p orbitals are present in each shell from the second row onward. 23 Where is the p-block on the periodic table.
L 1 p-subshell. Correct option is A Number of orbitals 2 l 1 For p sub shell l 1. 2 The number of orbitals present in an electron subshell is always six.
Number of orbitals in each subshell 2l 1 So Number of orbitals in h subshell 2times 5 1. They are dxy dyz dzx dx2-y2 dz2 n 6 l value 0 to n-1 l 012345 l. 5 orbitals for n 4 and l 2.
Next the p subshell has 6 electrons. D-subshell contains five orbital. 3 An atom as a whole is electrically neutral because all charged subatomic particles reside in asked Jun 25 2017 in Chemistry by littleone.
Answer verified by Toppr. The values of l correspond to. What is the maximum number of.
11 How many orbitals are there in a subshell for which N 4 and L 3. 12 How many orbitals are there combined for the 3d and 4d Subshells. 10 How many electrons are in a 4d orbital.
The subshell contains five orbitals for a similar reason. How many orbitals are present in a p - s. How many orbitals are present in a p subshell.
The notation describes the energy levels orbitals and the number of electrons in eachFor example the electron configuration of lithium is 1s 2 2s 1The number and letter describe the energy level and orbital and the number above the orbital shows how many electrons are in that orbital. You can determine how many orbitals the g-subshell would have by using quantum numbers. L 0 s-subshell.
9 Which of nodes in a 4d orbital is. 10 How many p orbitals are in the principal energy level. How many orbitals are in 1s.
Answer 1 of 3. 9 How many number of maximum electrons have quantum number n 4 ms in an atom. How many orbitals are there in the 3d subshell quizlet.
There are 4 subshells s p d and f. The s-orbital has one orbital ml 0 the p-subshell has three orbitals ml -1 0 1 and the d-subshell has 5 orbitals ml -2 -1 0 1 2. 14 How many electrons with N 5 can an atom contain.
7 How many 5d orbitals are there. 11 Do all 3d orbitals have the same shape. How many orbitals are present in the subshell with n 4 L 2.
The magnetic quantum number indicates the number of orbitals in a subshell. How many atomic orbitals are there in a p subshell. L 2 d-subshell.
Which means that the p subshell has 3 orbitals. 8 How many electrons can p orbitals. Experts are tested by Chegg as specialists in their subject area.
Maximum 6 electrons in 3 orbitals. 10 How many electrons maximum can have N 14 in the atom. Each subshell can hold a different number of electrons.
A hydrogen atom with one electron would be denoted as 1s1 -. 9 How many p orbitals are in the N 3 shell. What is the maximum value of the secondary quantum number C allowed for atomic electrons with a principal quanturn number n 7.
13 What orbitals are in the N 4 shell. Electron density distributions in space and energies eg. The number of orbitals depends on the max number of electrons within a subshell.
Also know why does P Subshell have 3 orbitals. Number of orbitals in a sub shell 2l1e 0 for s sub shell number of orbitals in s sub shell 201 1. 21 How many sublevels are there in the first energy level.
12 How many possible orbitals are there for N 3. The individual orbitals are. S p d f and so on are the names given to the orbitals that hold the electrons in atoms.
What is the total number of orbitals in the fourth energy level n 4. The formula to calculate the number of orbital in each subshell. 1 The wording orbital subshell The correct math but wrong physical model of the counter energy at an extra 1r comes from the Nels Bohr model which thought that math was a mechanical equation by the same concept as a gravitational orbit so orbital angular momentum wh.
7 How many orbitals are in p. 8 How do you draw a 4d orbital. 5 Does the second shell contain s and p orbitals.
These orbitals have different shapes eg. View the full answer. The angular momentum quantum number or l tells you the subshell in which an electron is located.

There Are 3 Orbitals In A P Subshell Ml 1 0 Or 1 Ppt Download

How Many Orbitals Are Present In P Subshell Youtube

Question Video Determining The Number Of Orbitals In An S Subshell Nagwa
0 Comments